DO NOT USE - ALL INFORMATION LIKELY INCORRECT IF NOT ACTIVELY DANGEROUS
Please use current guidelines available on the UHNM intranet for patient treatment
Please use current guidelines available on the UHNM intranet for patient treatment
BACKGROUND
- 4-factor PCC is a manufactured plasma product containing clotting Factors II, VII, IX and X, plus the natural anticoagulant proteins C and S
- Available as Octaplex® 500 IU or 1000 IU coagulation factor IX
- Store in controlled temperature <25°C for <2 yr
- Once requested keep in controlled storage at 2-8°C until required
- Only use PCC where clinically indicated as administration may exacerbate underlying pro-thrombotic states
- There is small risk of disseminated intravascular coagulation (DIC), particularly with repeated dosing
- Clinician direct access from the transfusion laboratory is available for agreed indications to ensure prompt treatment provision in recognised indications - [(RSUH: 0900–1700 hr call 74948 or out-of-hours bleep 390) (County: 0900–1700 hr call 4758 or
- for further details and relevant SOPs see Trust policy C03 or Blood and blood products intranet page
INDICATIONS
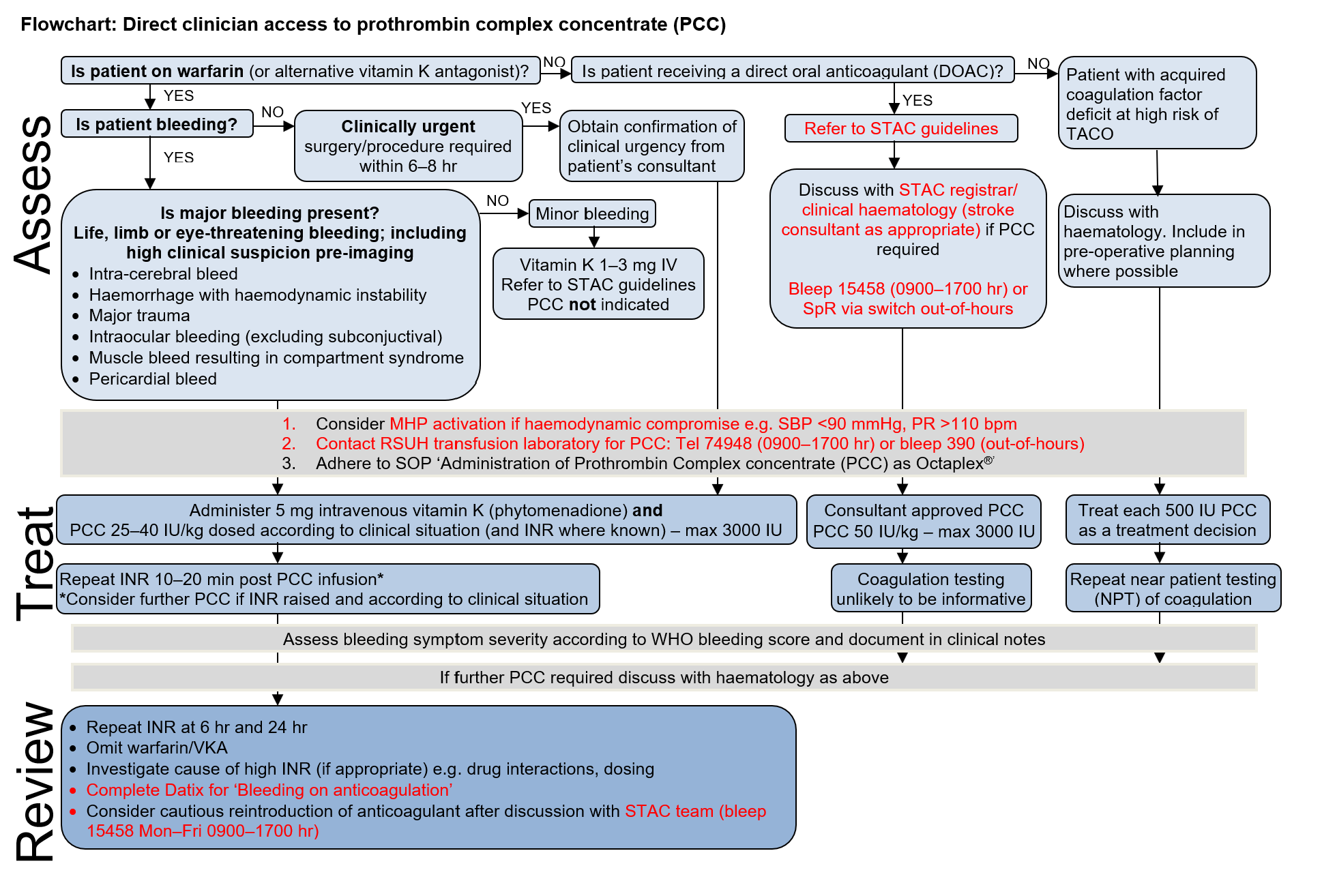
- Treatment of patients receiving warfarin or alternative vitamin K antagonists (VKA) experiencing major bleeding i.e. life, limb or eye-threatening bleeding. Includes high clinical suspicion of major haemorrhage pre-imaging
- Patients receiving warfarin or VKA requiring surgery or invasive procedure within the next 6-8 hr, due to clinical urgency only
- May be indicated for patients with major bleeding/pre-operatively receiving direct oral anticoagulants (DOACs) apixaban, rivaroxaban, edoxaban – see Bleeding in patient receiving DOAC guideline and seek advice from consultant haematologist (see STAC guideline 'Management of Bleeding in Patients on Antithrombotic Therapy')
- May be indicated for patients with other acquired coagulopathies (e.g. liver disease, cardiac surgery) where there is high risk of transfusion associated circulatory overload (TACO) - seek advice from consultant haematologist
CONTRAINDICATIONS
- Hypersensitivity to the active substance or any of the excipients (see SPC)
- Known allergy to heparin or history of heparin induced thrombocytopenia (HIT)
DOSE
- Dosed in 'international units' (IU) as multiples of 500 IU
- Maximum single dose 3000 IU (120 mL)
For anticoagulant reversal
- Dosed at 25-50 IU/kg according to patient weight and INR (where known) as advised by transfusion laboratory SOP (see Table 1 and 2, plus flowchart below)
- Do not await INR or imaging if high clinical suspicion of major haemorrhage - especially if suspected intracranial bleeding
- For warfarin reversal always ensure vitamin K (phytomenadione) 5 mg IV has been prescribed and administered - as PCC immediately (but only temporarily) reverses the anticoagulant effects of warfarin
- Ensure anticoagulant has been omitted
- Repeat INR 10-20 min post administration (see below re assessing response)
Table 1: PCC dose if major bleeding or urgent surgery/procedure but valid INR not yet available
| Weight (kg) | PCC dose (25 units/kg) |
|---|---|
| ≤60 | 1500 units |
| 61-80 | 2000 units |
| 81–100 | 2500 units |
| >100 | 3000 units |
Table 2: PCC dose if major bleeding or urgent surgery/procedure plus INR available and valid (i.e. taken within 8 hr and assess possible impact of previous vitamin K use)
| INR | Weight (kg) | PCC issue | PCC dose |
|---|---|---|---|
| 1.6–1.9 | n/a | 500 iu | |
| 2.0–3.5 | ≤60 | 1500 units | 25 units/kg |
| 61–80 | 2000 units | ||
| 81–100 | 2500 units | ||
| >100 | 3000 units | ||
| 3.6-5.0 | ≤60 | 2000 units | 33 units/kg |
| 61–75 | 2500 units | ||
| >75 | 3000 units | ||
| >5.0 | ≤60 | 2500 units | 40 units/kg |
| >60 | 3000 units |
As low volume FFP alternative
- Treat each 500 IU PCC as a treatment decision and evaluate clinically ± near patient testing (NPT) of coagulation post dose
- 1 IU PCC has equivalent clotting factor activity to 1 mL plasma (500 IU approximately equivalent to 2 units FFP)
ADMINISTRATION
- Commence infusion at 1 mL/min and observe closely for allergic reactions/anaphylaxis
- In major bleeding increase rate to 8-10 mL/min under direct clinical instruction
- Pre-surgery/procedure increase rate to 2-3 mL/min
- Return unused PCC to transfusion laboratory as soon as possible to avoid wastage
ASSESSING RESPONSE TO TRANSFUSION
- Post PCC administration, assess and document bleeding symptom severity according to WHO Bleeding score
- For warfarin reversal - repeat INR 10–20 min post PCC administration
- If adequate correction, recheck clotting after 4-6 hours then daily
- If INR ≥1.5 or suboptimal correction and further PCC may be required – seek advice from a consultant haematologist
- Monitor for adverse events of PCC usage - especially thrombosis
- Complete Datix where indication is 'major bleeding on anticoagulation' and discuss with STAC registrar on 15458
Last reviewed: 2024-04-29