DO NOT USE - ALL INFORMATION LIKELY INCORRECT IF NOT ACTIVELY DANGEROUS
Please use current guidelines available on the UHNM intranet for patient treatment
Please use current guidelines available on the UHNM intranet for patient treatment
BACKGROUND
- To revise the principles of transfusion, see Principles of transfusion and Consent for transfusion guidelines
- Packed red blood cells (RBC) in SAG-M additive solution, 280 ± 60 mL, Hct 0.5-0.7
- Collected from UK volunteer whole blood donors i.e. allogeneic
- Stored in controlled temperature at 2-6°C for <35 days
- Only store red cells in designated blood fridges
- Allocated RBC routinely derequisitioned (i.e. returned to stock) at 24 hr
- Areas without a satellite fridge collect 1 unit of blood at a time
- except renal dialysis patients and MHP activation
INDICATIONS
- Use red cells to restore oxygen carrying capacity in patients with anaemia or blood loss where alternative treatments are ineffective or inappropriate
- Base decision to transfuse on the whole clinical picture:
- cause of anaemia
- symptom severity
- underlying co-morbidities
- chronicity
- current and historic laboratory parameters
- RBC transfusion is not associated with reduced mortality. Base decision to transfuse patients (with a single unit followed by reassessment) on the need to relieve clinical signs and symptoms of anaemia, alongside the patient’s response to previous transfusions
Acute blood loss with haemodynamic instability/uncontrolled haemorrhage
- Benefit: Save life
- Target Hb: 70-90 g/L (once haemodynamically stable)
- Blood loss of >20-30% (where average circulating blood volume is 70 mL/kg) with on-going bleeding will likely require urgent transfusion
- Use cell salvage where possible to minimise allogeneic transfusion requirements
- See specific guidelines, including Acute upper gastrointestinal haemorrhage guideline and Major haemorrhage pathway
Recoverable anaemia in a haemodynamically stable patient e.g. post op, critical care
- Benefit: improve symptoms of anaemia, potentially reduce mortality
- Target Hb: 70-90 g/L (once haemodynamically stable)
- Threshold Hb for transfusion: <70 g/L
- Note: Do not transfuse stable patients with iron deficiency anaemia. Give IV iron. See Iron deficiency guideline
- Note: Do not transfuse stable patients with B12 and/or folate deficiency - see Chronic anaemia guideline, B12 deficiency and Folate deficiency guidelines
- Note: No evidence was found to warrant a different approach for patients who are elderly or who have respiratory or cerebrovascular disease - who may also be at increased risk of fluid overload from transfusion
- Note: Use higher threshold of 75g/L in cardiac surgery patients (during and after surgery)
Acute coronary syndrome (ACS)
- Benefit: improve short term outcome
- Target Hb: 80-100 g/L (once haemodynamically stable)
- Threshold Hb for transfusion: <80 g/L
- Note: Transfusion >100 g/L associated with increased mortality
Chronic transfusion dependence e.g. bone marrow failure (MDS, thalassaemia)
- Benefit: improve quality of life, reduce extramedullary haemopoiesis in thalassaemia
- Target Hb: individual to patient (depending on cause and response)
- Threshold Hb for transfusion: start at 80 g/L and adjust as required
Radiotherapy (weak evidence)
- Benefit: improved response to therapy
- Target Hb: individual to patient
- Threshold Hb for transfusion: consider if <100 g/L in cervical cancer and other cancers receiving radiotherapy
- Note: In patients with cancer, RBC transfusion may be associated with an increased risk of in-hospital mortality, plus increased risk of venous and arterial thromboembolic events
Exchange transfusion e.g. sickle cell disease following acute stroke/chest crisis, or before surgery; haemolytic disease of the fetus and newborn (HDFN)
- Benefit: replace red cells and treat/prevent symptoms
- Target Hb: individual to patient
- Threshold Hb for transfusion: n/a
- Note: See regional sickle cell transfusion guidelines or neonatal guidelines
CAUTIONS
- Regard each unit of red blood cells transfused as a treatment decision
- Blood transfusion is associated with significant risk
- use alternatives to transfusion wherever possible and appropriate
- Use the minimum number of units required to achieve target Hb/relieve moderate-severe symptoms i.e. single unit transfusion policy
- Patients with acute coronary syndrome (80 g/L), cardiac surgery (75 g/L), orthopaedic surgery and haemato-oncology patients and may require higher targets
- Except in circumstances where patient’s condition is life-threatening, patient must be given time to ask questions and to make a decision to proceed with transfusion
- Always document indication for transfusion and consent in the medical notes
ALTERNATIVES TO TRANSFUSION
- Treat underlying cause of anaemia e.g. haematinic deficiency (see relevant medical guidelines)
- Minimise and treat blood loss e.g. surgical techniques, endoscopy, hormonal treatment of heavy menstrual bleeding, anti-fibrinolytics
- Uphold restrictive transfusion thresholds where appropriate, maximise oxygenation and optimise management of underlying medical conditions
- Use cell salvage to minimise allogeneic transfusion where possible
- Employ normovolaemic haemodilution/permissive hypotension where appropriate
- Minimise iatrogenic anaemia by reducing phlebotomy and consider use of paediatric bottles
PRESCRIPTION/AUTHORISATION
- Each single unit RBC transfused is a treatment decision (except in active bleeding) = SINGLE UNIT TRANSFUSION POLICY
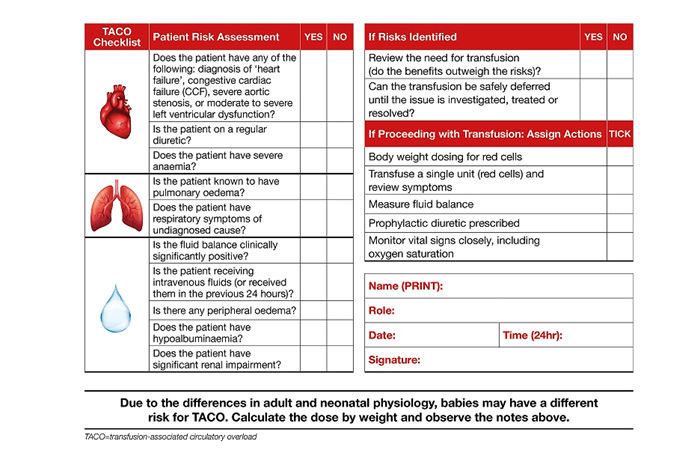
- Before every transfusion, assess all patients for risk of transfusion associated circulatory overload (TACO) - see TACO checklist below
- manage appropriately e.g. slow infusion rate, diuretic use, increase frequency of observations
- Prescribe red blood cells (RBC) on fluid prescription of the drug chart (or specific transfusion chart where available)
- dose in units (or mL in low weight patients e.g. <50 kg at high risk of TACO)
- indicate special blood requirements (SBR) e.g. irradiated, HbS neg, Rh/K matched on prescription or specify 'no SBR' (just as important)
- Specify transfusion rate depending on clinical situation (do not give a range on prescription chart)
- if low risk of TACO, 120 min per unit
- if high risk of TACO, 3 hr per unit ± diuretics
- if MHP, 'STAT' through blood warmer (i.e. over 5-10 min - rate will depend on bleeding severity)
- In the absence of active bleeding, use minimum number of units required to achieve target Hb taking into account patient size
- 1 unit RBC expected to raise Hb by 10g/L in 70 kg patient (but note 1 unit = 220-340 mL)
- 4 mL/kg RBC expected to raise Hb by 10g/L (use in adult patients <50 kg)
- or use online dosage calculator https://www.rcdcalculator.co.uk/
TACO Checklist
ADMINISTRATION
- Commence transfusions as soon as possible after component receipt in the clinical area
- Complete transfusion within 4 hr of RBC leaving cold storage
- In areas without a satellite blood fridge, only one unit of blood per patient to be collected at a time (except renal dialysis patients and MHP activation)
- Any unused units to be returned (on availability and location system) to the transfusion laboratory (or designated blood fridge) ASAP to avoid wastage
- ideally within 30 min of leaving controlled temperature storage
- Use standard blood giving set with 170-200 micron filter
- Adhere to standard operating procedure for administration of blood components regarding bedside checks and monitoring frequency
- Any blood component connected to the patient’s IV access is regarded as 'transfused' for traceability purposes - even if the unit was subsequently (partially) wasted
- Blood warmers to be used if clinically significant cold antibodies, elective/emergency surgery requiring ≥500 mL of fluids including blood components, major haemorrhage
- Complete transfusion within 4 hr of red blood cells leaving cold storage
ASSESSING RESPONSE TO TRANSFUSION
- Each single unit RBC transfused is an independent treatment decision
- Assess every patient clinically after each unit transfused asking;
- have symptoms/signs of anaemia resolved? - document severity grade (see Table 1)
- is there evidence of fluid overload (TACO)? - document any symptoms/signs
- what is the Hb increment after each unit transfused?
- Note: Check Hb after each unit transfused except in active bleeding, chronically transfused outpatients, or where target threshold cannot realistically be achieved.
- In active bleeding (haemodynamically stable patients) check Hb increment after maximum 2 unit RBC transfusion to avoid over-transfusion
- note: repeat Hb can be performed from 15 min post transfusion as FBC or blood gas (latter for response assessment only - not initial treatment decision)
- Patients transfused to >20 g/L above target threshold are deemed 'over-transfused'
- Ensure definitive treatment also prescribed e.g. iron supplementation where appropriate
Table 1: Anaemia severity grading score
| Severity score | Anaemia symptoms |
| Mild | Fatigue, shortness of breath on exertion |
| Moderate | Shortness of breath at rest, palpitations |
| Severe | Chest pain, symptoms of heart failure |
Document
- Fully document consent for transfusion, efficacy/impact and any complications in medical and nursing notes
- If moderate or severe acute transfusion reaction - complete Datix
- In discharge letter, document any transfusion (including component details, dates and any complications)
- Specify if definitive treatment also prescribed/given (where appropriate)
EMERGENCY RED CELLS
- Group O RhD negative blood cells are a finite resource – use only where clinically
indicated i.e.
- Group O RhD negative patients
- major haemorrhage in women of child-bearing potential
- life-threatening haemorrhage whilst awaiting arrival of group O appropriate red cells
- See major haemorrhage policy
Administration
- Take crossmatch sample before group O red cell administration
- 2-sample rule does not apply in emergency setting
- Switch to group specific red cells as soon as available
Access
- Only staff who have undergone appropriate fridge training can access O RhD negative units (barcode required)
- Hospital specific method of access
Last reviewed: 2024-04-15